NH3 is a weak base (Kb = 1.8 times 10^-5) and so the salt NH4Cl acts as a weak acid. What is the pH of a solution that is 0.050 M in
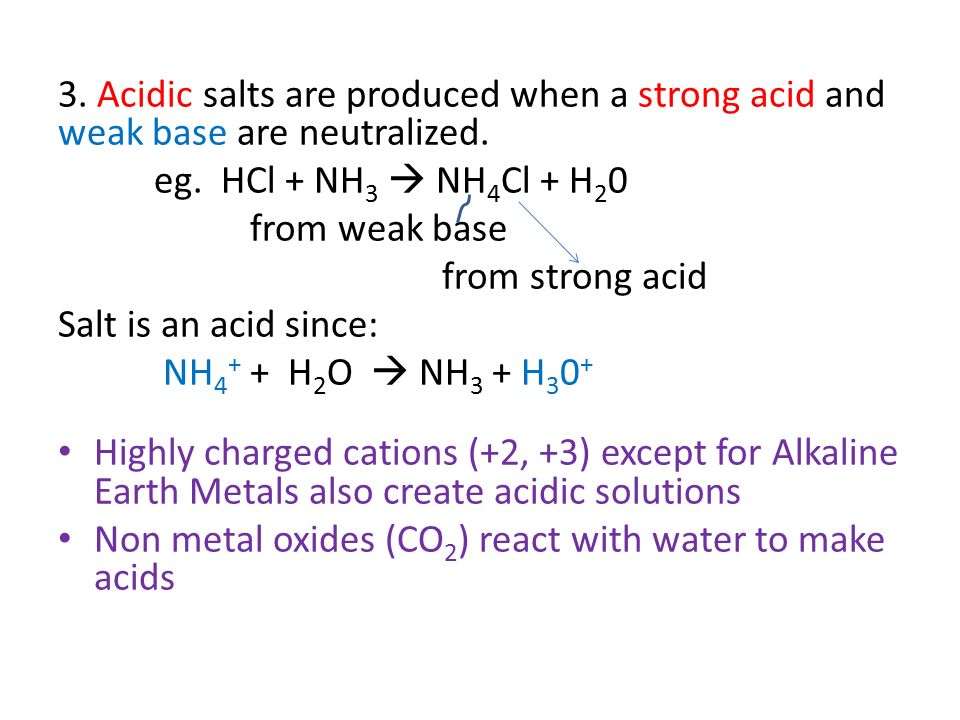
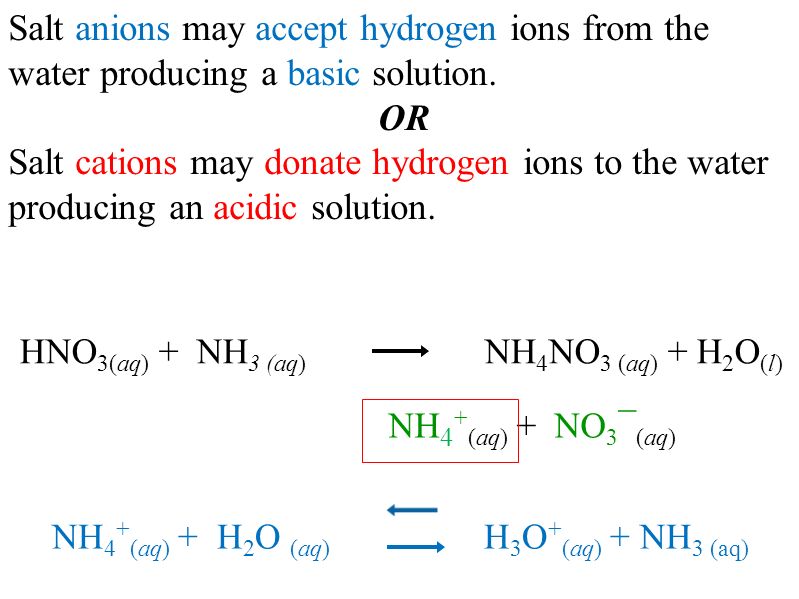
PH of Salts When acids are neutralized by bases, water and a salt is formed. The salt solution can be acidic, basic, or neutral depending on the acid. - ppt download
![Qualitative Analysis – Identification of Cations using aqueous Ammonia [Online Video] – O Level Secondary Chemistry Tuition Qualitative Analysis – Identification of Cations using aqueous Ammonia [Online Video] – O Level Secondary Chemistry Tuition](https://icandochemistry942105908.files.wordpress.com/2019/03/f9097-qa-cations-aqueous-ammonia.jpg?w=640)
Qualitative Analysis – Identification of Cations using aqueous Ammonia [Online Video] – O Level Secondary Chemistry Tuition
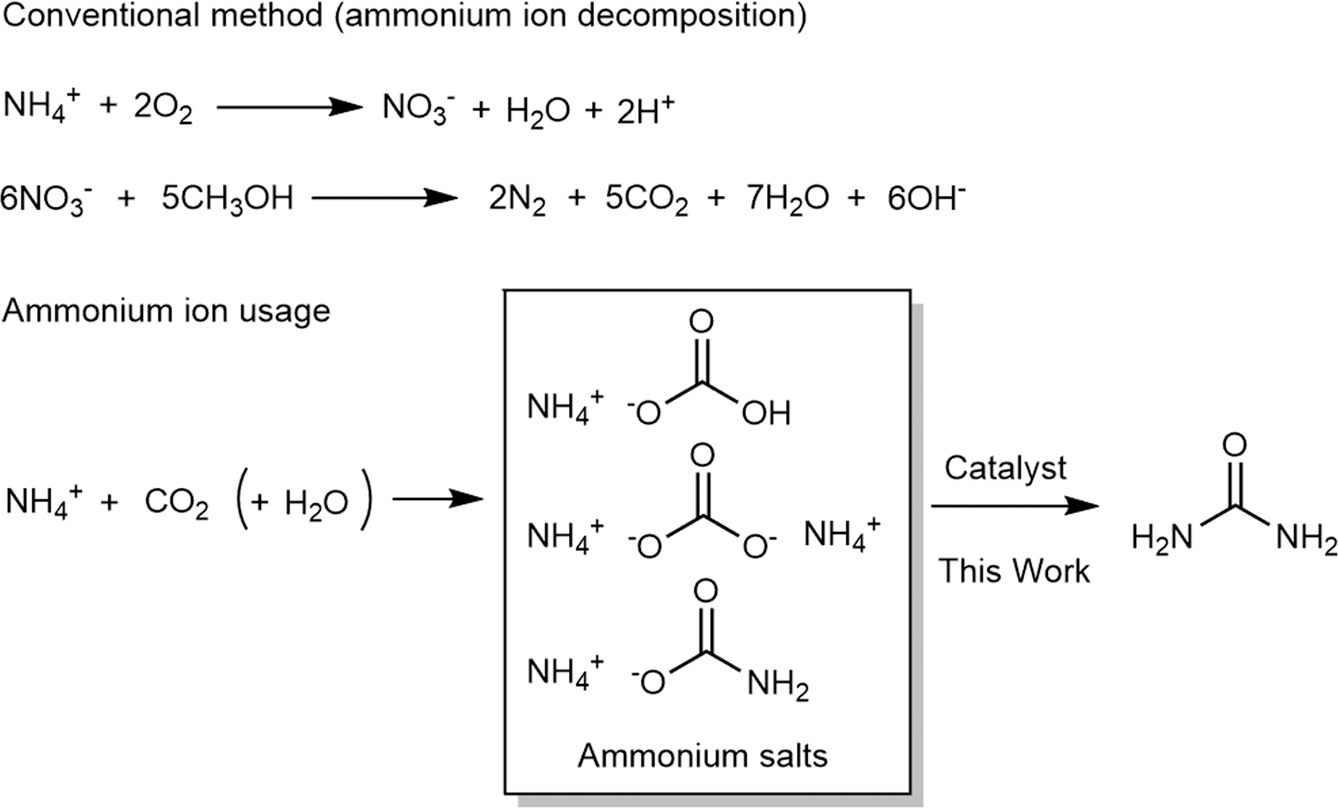
Organic bases catalyze the synthesis of urea from ammonium salts derived from recovered environmental ammonia | Scientific Reports

Ammonium salt production in NH3-CO2-H2O system using a highly selective adsorbent, copper hexacyanoferrate - ScienceDirect

NH3 is a weak base (Kb = 1.8 times 10^-5) and so the salt NH4Cl acts as a weak acid. What is the pH of a solution that is 0.050 M in

Smelling Salts for Athletes - Squeeze & Sniff! Pre-Activated Salt with Hundreds of Uses Per Bottle - Powerlifting Ammonia Inhalant - Rush, Alert Supplement - Inhalants for Fainting - by AmmoniaSport :
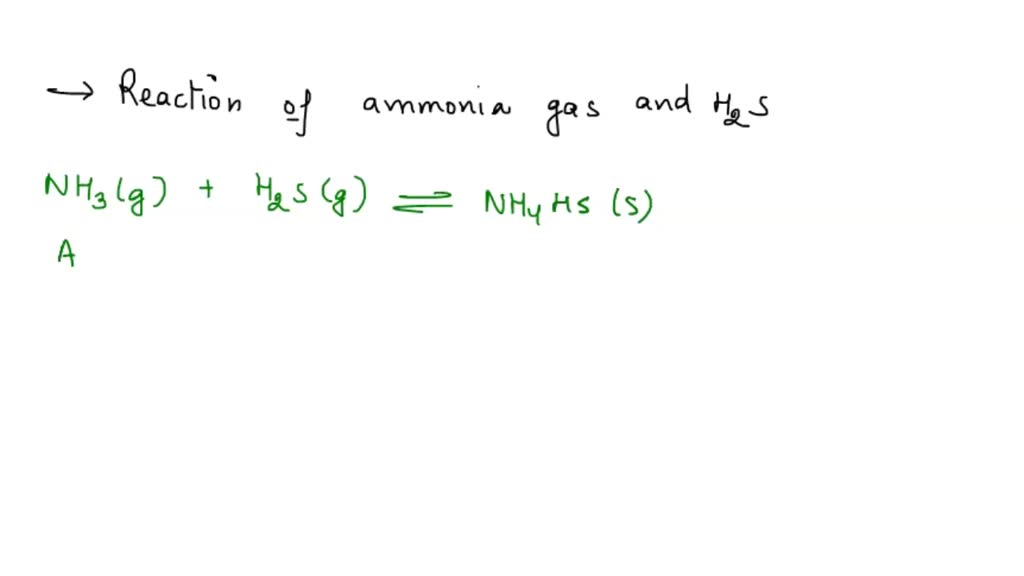
SOLVED: Ammonia gas NH3 and hydrogen sulphide gas H2S react together to form a salt ammonium sulphide ammonium sulphide dissolves in water to produce and orange alkaline solution.The addition of NaOH (aq)


.png)